
What is Graphite?
What is Graphite And Where Does Graphite Come From?
 We’ve written a lot in this blog about our primary material, graphite. So what is graphite? The word graphite comes from the Greek word graphein, which means "write" (due to it's use as pencil lead). As for the molecular structure, Graphite is made from carbon atoms, which have four valence electrons.
We’ve written a lot in this blog about our primary material, graphite. So what is graphite? The word graphite comes from the Greek word graphein, which means "write" (due to it's use as pencil lead). As for the molecular structure, Graphite is made from carbon atoms, which have four valence electrons.
While natural graphite occurs primarily in metamorphic rock mined in many countries from South America to Eastern Asia, we want to clarify that when we talk about graphite in most industrial applications, we are discussing synthetic graphite (so this is the last you will hear about metamorphic rocks!).
Synthetic graphite is the crystalline solid form of carbon. It is a man-made material that is extremely resistant to high temperatures and acidic or basic solutions. Graphite can be engineered to obtain specific properties such as density, electrical conductivity, hardness, porosity, compressive strength, flexural strength, coefficient of thermal expansion, and thermal conductivity.
From nuclear reactors to precious metal ingot molds to lithium ion batteries and other fuel cells, graphite is a versatile material with a number of uses cases due to it's variety of potential physical properties.
Below, we will detail the processes that go into making synthetic graphite and the different forms it can take, such as extruded graphite and isostatic graphite while answering the questions what is graphite? and where does graphite come from? Read on if you can take the heat!
How is Graphite Produced?
Typically, graphite is produced from petroleum coke which is heated to incandescence, which drives off many volatiles. The coke is then crushed and ground to specific particle sizes dictated by the desired final grade. The coke powder is mixed with coal tar pitch and other additives which act as binders. This mixture can be extruded or molded into desired blocks and rounds.
There are various methods of producing synthetic graphite shapes. The most commonly used methods are extrusion, compression molding, and isostatic molding.
The Baking Cycle: 60 Days to Solid Carbon
Once the "green" or raw carbon blocks are molded they undergo an extended baking cycle to convert the pitch into solid carbon. This process may take up to 60 days and is carefully controlled to prevent the material from fracturing.
Graphitization: Turn Up The Heat!
Once the baking cycle is complete the "baked carbon" is ready for the final process of graphitization. The conversion to graphite from baked carbon requires extremely high temperature. The temperature normally required for complete graphitization is 5,000 degrees Fahrenheit or higher. This temperature is typically reached in a controlled atmosphere induction furnace. An added benefit of the extremely elevated temperature is the expulsion of most of the impurities.
Your Final Product and Varying Graphite Grades
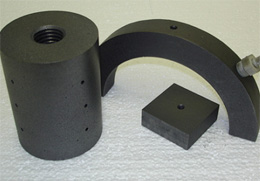 The result of the final graphitization process is a solid graphite block or round made up of graphite particles held together by the converted binder. The characteristics of the graphite are dictated by the recipe used which will specify the particle size, type of coke, final porosity, additives, and method of molding depending on the physical properties of the finished product.
The result of the final graphitization process is a solid graphite block or round made up of graphite particles held together by the converted binder. The characteristics of the graphite are dictated by the recipe used which will specify the particle size, type of coke, final porosity, additives, and method of molding depending on the physical properties of the finished product.
Typically, extruded graphite is a larger particle size (0.030-0.060") material which is used as a general purpose material, while isostatically molded graphite is composed of extremely small particles (4-10 micron). Isostatic graphite takes much longer to manufacture due to the extra steps required to mill the raw materials to a very fine and consistent powder prior to molding.
Extruded graphite is generally less than half the cost of the higher cost isostatic graphite, but the price difference reflects the characteristics obtained as the isostatically molded graphite has very low porosity, high density, consistent resistance, and high strength.
Diving Deeper: Synthetic Graphite's Crucial Key to the Resilient Industrial Machine
In the vast machinery that powers modern industry, synthetic graphite stands as one of the quieter, but no less significant, components. There are multiple roles that synthetic graphite plays within varied industrial sectors, showcasing its versatility and necessity in an array of manufacturing processes.
Let's dissect the crucial cogs of synthetic graphite in industries like diamond tool manufacturing, electroplating, heat treatment, induction furnaces, metal casting, and more.
Uncovering Synthetic Graphite's Role
At the very foundation of the industrial world lies the use of graphite. From high-precision engineering to the heat and electrical conductivity demands of multiple applications, graphite’s unique properties make it indispensable.
Synthetic graphite, in particular, has found a critical role in enhancing the performance and lifespan of various products. It often serves as a more consistent and reliable alternative to natural graphite, due to its highly engineered and controllable production process that ensures quality and a specific set of properties.
A Spark in Diamond Tool Manufacturing
The stakes are exceptionally high in this sector, where the use of synthetic graphite can be the difference between sharp precision and mediocre effectiveness. Diamond tooling manufacturers lean on synthetic graphite for its thermal management properties, form stability, and resistance to high-temperature degradation.
By collaborating with manufacturers like Semco Carbon, tool makers can tailor graphite's properties to their specific needs, ensuring that the end product stays on the cutting edge — quite literally.
Plating the Future with Graphite in Electroplating
Electroplating is a process where metal ions in a solution are moved by an electric field and deposited onto a conductive object. Graphite, with its electrically conductive property and resistance to chemicals, takes a vital role in this process.
Graphite is used to create anodes, cathodes, and other components needed for the precise and efficient transfer of metals such as chrome, nickel, and copper onto the substrate. The ability to customize graphite for specific electroplating processes positions it as an enabler of fine-tuned surface finishing across industries.
The Heat Treatment Ecosystem
In the world of heat treatment, graphite assumes the mantle of a high-temperature, non-metallic, and conductive material that's indispensable for furnaces and related processes. It finds applications in resistance heaters, furnace fixtures, and more, where its special grade and impregnations boast the ability to withstand the harshest thermal processing environments. Custom graphite solutions are key in maintaining precise heat treatment conditions, ensuring that materials are treated reliably and consistently.
Lighting the Way in Induction Furnaces
Induction furnaces leverage graphite's thermal conductivity to control the non-contact heating process, particularly in melting and casting metal operations. This includes everything from providing melting crucibles and pouring troughs to crafting molds that can withstand the rigors of induction heating. Synthetic graphite's capability to manage heat uniformly makes it a go-to material for these high-energy usage applications, contributing to the efficiency and integrity of the metal casting process.
Casting a Stronger Metal with Graphite Solutions
Graphite shines in metal casting as well, where it is used in the production of a variety of precision components such as crucibles, molds, and risers. Its resistance to thermal shock and its ability to maintain shape under extreme heat conditions make it ideal for casting ferrous and non-ferrous metals.
The result is a casting process that is not only efficient but also produces higher quality end products, critical in industries where material consistency is paramount.
In Pursuit of Precious Metals
 The term 'precious' extends to the precision required in the tools and technologies that handle metals like silver, gold, and platinum. Synthetic graphite fulfills this demanding need through the creation of complex casting systems components, such as split dies and heating elements, that facilitate the impeccable microstructure of these valuable materials, ensuring they are of the highest quality and integrity.
The term 'precious' extends to the precision required in the tools and technologies that handle metals like silver, gold, and platinum. Synthetic graphite fulfills this demanding need through the creation of complex casting systems components, such as split dies and heating elements, that facilitate the impeccable microstructure of these valuable materials, ensuring they are of the highest quality and integrity.
Illuminating the Solar and Crystal-Growing Industries
The demand for synthetic graphite extends to emerging sectors like solar and crystal-growing technologies. Here, its use as a heating element, along with the creation of fixtures and hot zone components, enables the controlled and stable growth of crystals and materials, particularly in the production of silicon wafers for photovoltaic cells.
The consistency provided by high-quality synthetic graphite is a critical part of the process that is pioneering sustainable energy solutions for the future.
The Sustainability Conundrum
While the utility of synthetic graphite is undeniable, manufacturers are increasingly being challenged to ensure that its production aligns with environmental sustainability goals. This is a complex issue, as synthetic graphite requires significant energy input for its high-temperature synthesis processes.
However, innovations such as the utilization of renewable energy sources and the development of more efficient production methods aim to make synthetic graphite a sustainable component of the industrial landscape.
Graphite for the Future
As technology continues to advance, so too does the role of synthetic graphite. Its applications will likely expand into new frontiers, driven by our collective need for reliable and high-performance materials. Looking ahead, the challenge remains not just in finding new uses for synthetic graphite, but also in innovating its production and recycling to meet the triple-bottom-line demands of modern industry.
From enhancing the efficiency of solar panels to enabling the precision in the gears of our industrial progress to simple and complex needs for thermal insulation, synthetic graphite is set to be a crucial player for the foreseeable future. The ongoing quest to balance its indispensable use with the planet's well-being will push manufacturers and innovators alike to tread the line between progress and preservation.
Here lies the next frontier for the resilient industrial machine, with synthetic graphite as its silent, but solid, foundation.
Contact Semco To Learn More!
When you contact Semco about your graphite needs, our graphite experts can guide you through choosing the material most appropriate for your application.
We’ve seen it all, including situations where a company tried to cut costs by using a lower-grade graphite when their set-up called for a higher grade material, only to have to replace an entire machine within months. Our knowledgeable engineers will provide you with an assessment that will help protect your investment.
Let us help you ensure that your graphite components operate correctly for the long haul.

